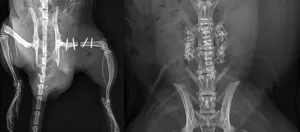
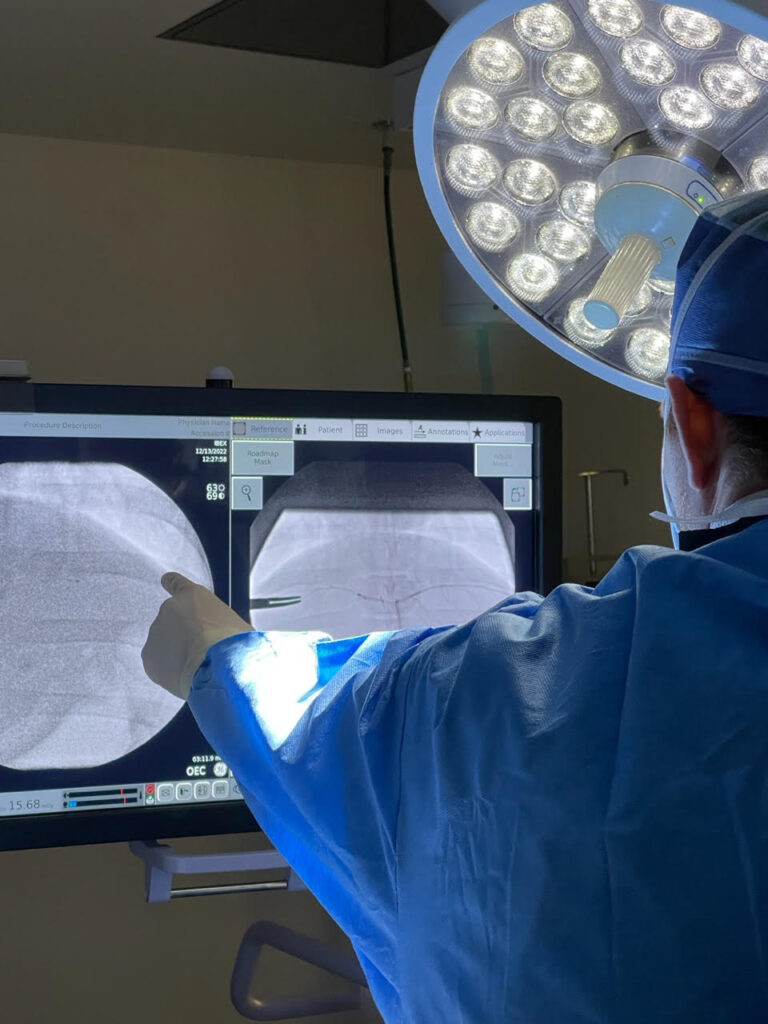
Interventional and minimally invasive instrument preclinical studies are essential for evaluating the safety, functionality, and effectiveness of devices used in procedures that require precision and minimal disruption to surrounding tissues. Using various animal models, researchers can test and refine devices like catheters, stents, and surgical instruments, ensuring they achieve the desired therapeutic outcomes without causing undue harm or complications.
Over the years, IBEX has built and maintained a team of exceptionally skilled veterinary surgeons, clinical consultants, and preclinical consultants who excel in supporting fast and verifiable research needs.
Whether you need custom device testing or strong documentation for regulatory submissions, IBEX has the capacity and expertise to get it done right.
Contact us today to book your consultation.
Ibex has performed hundreds of orthopedic surgical studies in a variety of laboratory animal species since 2003. Indeed, orthopaedics is one of our major areas of expertise. We commonly perform spine fusions, drill defects and long bone segmental defects in a variety of species, but we also are experienced with flat bone and mandibular defects studies. We perform efficacy studies that mimic clinical implantation, regulatory (ISO10999) biocompatibility studies (ISO) and discovery or due diligence studies. Flat bone defects (rat and rabbit craniotomy) are also performed. Our systems are well-honed to be efficient, so clients attending the procedures can get in and out quickly. Surgical procedures are performed by an experienced veterinary surgeon, resulting in surgical quality, reproducibility and uniform techniques between cases and studies. Our clients have a variety of study objectives, ranging from ISO 10999 biocompatibility, implant toxicity, efficacy or internal screening due diligence studies.